My research includes using mathematical modeling to understand physiological processes, in particular mathematical ophthalmology. Currently my work is expanding from dynamic mathematical and computational multi-scale models of cellular and molecular processes to include experimental work that complements current retina degeneration research, in particular retinitis pigmentosa (RP) and age-related macular degeneration (AMD). My work to-date includes mathematical models of key metabolic pathways in cones (particularly aerobic glycolysis) and glutathione redox system, photoreceptors and RPE interactions, and treatments to slow photoreceptor degeneration. My research investigates both the healthy and diseased retinas at the cellular and molecular levels and disruptions in the photoreceptors' metabolic processes resulting from nutrient deprivation, increased oxidative stress, and oxygen imbalance. This research will help identify pathways and mechanisms within the photoreceptors and retinal pigmented epithelium (RPE) that can help prevent or slow down their degeneration, including those involved in metabolic adaptation, redox balance restoration, and protective stress responses in diseased and aging retinas, and generating data to develop and complement dynamic mathematical models. My mathematical models, calibrated with real-world data, provide new insights into methods that can reduce vision impairment.
My research interests center on 1) the metabolic needs of cones in the absence of rods, before, during and after degeneration and retinal remodeling, 2) aerobic glycolysis and oxidative stress pathways in photoreceptors and the retinal pigment epithelium (RPE), 3) metabolic pathways implicated in photoreceptor degeneration, and 4) immune response in retinal degenerative diseases.
Fulbright Award Testimonial, 2023
Fulbright Scholar at L'Institut de la Vision
As a Fulbright scholar and visiting scholar conducting research at the Institut de la Vision (IDV) in Paris in 2022–2023, I am investigating pathways in the rods and cones that may help us understand how cones and rods developed (in an evolutionary sense) to the interdependent system that we see today.
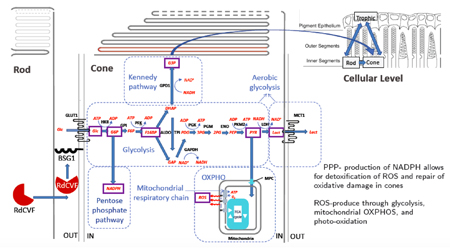
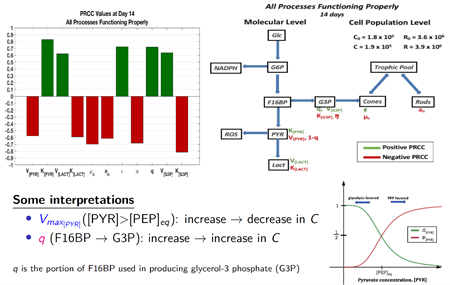
Camacho, et al., 2019. A mathematical analysis of aerobic glycolysis triggered by glucose uptake in cones. Scientific reports, 9(1).
Cone Photoreceptors
Cone photoreceptors are one of the most energy-demanding cells in the body and are responsible for day vision and acuity. They produce their energy through aerobic glycolysis, which is the same process used by cancer cells. We model aspects of aerobic glycolysis and examine pathways in an effort to better understand key mechanisms in this important process.
Some publications:
- Dobreva, A., Camacho, E.T., Larripa, K., Rǎdulescu, A., Schmidt, D.R. and Trejo, I., 2022. Insights into pathological mechanisms and interventions revealed by analyzing a mathematical model for cone metabolism. Bioscience Reports, 42(3), p.BSR20212457.
- Camacho, E.T., Dobreva, A., Larripa, K., Rǎdulescu, A., Schmidt, D. and Trejo, I., 2021. Mathematical modeling of retinal degeneration: aerobic glycolysis in a single cone. Using Mathematics to Understand Biological Complexity: From Cells to Populations, pp.135-178.
- Camacho, E.T., Brager, D., Elachouri, G., Korneyeva, T., Millet-Puel, G., Sahel, J.A. and Léveillard, T., 2019. A mathematical analysis of aerobic glycolysis triggered by glucose uptake in cones. Scientific Reports, 9(1), p.4162.
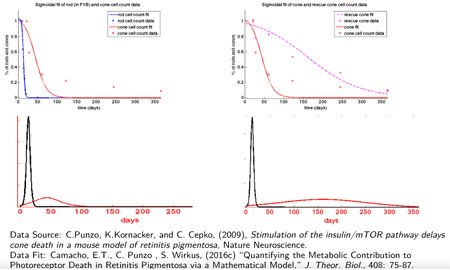
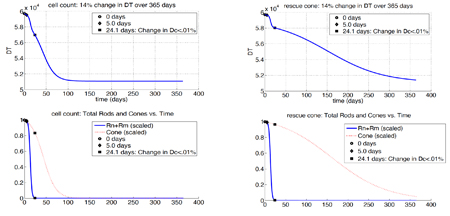
Camacho, Punzo, & Wirkus, 2016. Quantifying the metabolic contribution to photoreceptor death in retinitis pigmentosa via a mathematical model. Journal of Theoretical Biology, 408.
Photoreceptor Degeneration and Retinitis Pigmentosa
Retinitis pigmentosa (RP) is a term used to describe the heterogeneous group of inherited blindness. While most mutations occur in the rod photoreceptors, the progression of the disease is similar regardless of the mutation–the rods begin to die and when most have been lost, the cones then begin to die. As rods are responsible for night vision and peripheral vision in humans, many people don't report problems soon enough. There is currently no cure for RP and mathematical models provide insight into ways that may slow the loss of photoreceptors.
Some publications:
- Wifvat, K., Camacho, E.T., Kawski, M., Léveillard, T., and Wirkus, S., 2024. Optimal Control with RdCVFL for Degenerating Photoreceptors. Bulletin of Mathematical Biology, 86(3): 29.
- Camacho, E.T., Lenhart, S., Melara, L.A., Villalobos, M.C. and Wirkus, S., 2020. Optimal control with MANF treatment of photoreceptor degeneration. Mathematical Medicine and Biology: A Journal of the IMA, 37(1), pp.1-21.
- Camacho, E.T., Punzo, C. and Wirkus, S.A., 2016. Quantifying the metabolic contribution to photoreceptor death in retinitis pigmentosa via a mathematical model. Journal of Theoretical Biology, 408, pp.75-87.
- Camacho, E.T., Melara, L.A., Villalobos, M.C. and Wirkus, S., 2014. Optimal control in the treatment of retinitis pigmentosa. Bulletin of Mathematical Biology, 76, pp.292-313.
- Camacho, E.T. and Wirkus, S., 2013. Tracing the progression of retinitis pigmentosa via photoreceptor interactions. Journal of Theoretical Biology, 317, pp.105-118.
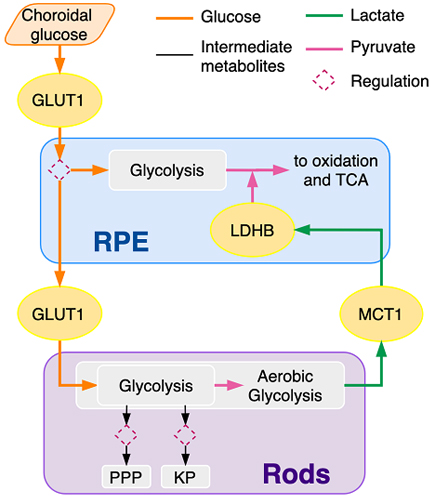
Aparicio, Camacho, et al. 2022. A mathematical model of GLUT1 modulation in rods and RPE and its differential impact in cell metabolism. Scientific reports, 12(1).
Math Models of Photoreceptor Interactions
The rod and cone photoreceptors together with the retinal pigmented epithelium (RPE) function as a critical and interdependent unit in the vision process. Failures in any one of the them can have a cascading effect on all of them. We seek to mathematically model the processes that make this unit work in order to gain insight into their failures.
Some publications:
- Aparicio, A., Camacho, E.T., Philp, N.J. and Wirkus, S.A., 2022. A mathematical model of GLUT1 modulation in rods and RPE and its differential impact in cell metabolism. Scientific Reports, 12(1), p.10645.
- Wifvat, K., Camacho, E.T., Wirkus, S. and Léveillard, T., 2021. The role of RdCVFL in a mathematical model of photoreceptor interactions. Journal of Theoretical Biology, 520, p.110642.
- Camacho, E.T., Léveillard, T., Sahel, J.A. and Wirkus, S., 2016. Mathematical model of the role of RdCVF in the coexistence of rods and cones in a healthy eye. Bulletin of Mathematical Biology, 78, pp.1394-1409.
- Camacho, E.T., Vélez, M.A.C., Hernández, D.J., Bernier, U.R., Van Laarhoven, J. and Wirkus, S., 2010. A mathematical model for photoreceptor interactions. Journal of Theoretical Biology, 267(4), pp.638-646.
- Vélez, M.A.C., Hernández, D.J., Bernier, U.R., Van Laarhoven, J. and Camacho, E.T., 2003. Mathematical models for photoreceptor interactions. Cornell University, Dept. of Biological Statistics & Computational Biology, Technical Report, BU-1640-M.
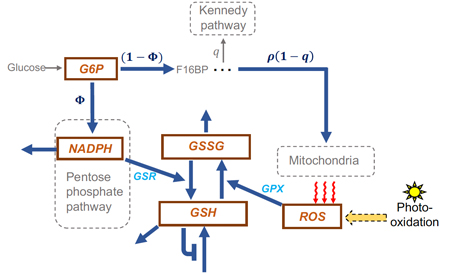
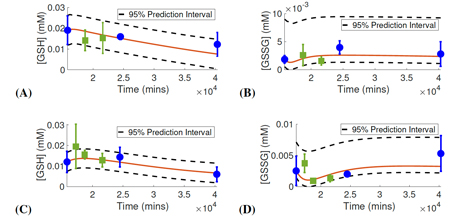
Dobreva, A., Camacho, E.T., and Miranda, M., 2023. Mathematical model for glutathione dynamics in the retina. Scientific reports, 13(1), p.10996.
Retina Redox system
The retina is highly susceptible to the generation of toxic reactive oxygen species (ROS) that disrupt the normal operations of retinal cells. Redox systems are critical in sustaining homeostasis, maintaining normal retina functioning, and preventing retinal degenerative processes. Excessive ROS can cause damage to various retina mechanisms and components, including DNA, lipids, and proteins, triggering abnormal redox signaling and inflammatory responses, and ultimately lead to cell death.
Some publications:
- Dobreva, A., Camacho, E.T., and Miranda, M., 2023. Mathematical model for glutathione dynamics in the retina. Scientific Reports, 13(1), p.10996.
Selected Grants Supporting Research
Retina Research Foundation — "Reducing Retinal Blindness Worldwide" 
https://retinaresearchfnd.org/pilot-study-grants/grant-recipients/erika-tatiana-camacho-phd/
Project goal: Modeling the role of 6-phosphofructo-2-kinase/fructose-2, 6 bisphosphatase 2 in retinal metabolism and retinal degenerative diseases
Interviewed for ASU ADVANCE Institutional Transformation program, Dr. Erika Tatiana Camacho briefly explain her research, academic trajectory, and passion for creating opportunities to advance STEM.
Links to some recorded research presentations
- The Role of Cone Metabolism in Mitigating Blindness, Jan 29, 2021, AWM 50th Anniversary Inaugural speaker, We Speak: Inspiring Women in Math Speaker Series.
- The Role of Cone Metabolism in Mitigating Blindness, Apr 30, 2021, Cornell University, CAM Notable Alumni Lecture Series Speaker.
- Modeling Photoreceptor Death and Rescue, April 2015, Latinos in Mathematics Conference, IPAM, CA.











