The recent chemistry performed in the Doyle Group has created convenient access to a vast array of new chemical structures that are otherwise difficult to prepare. They fall into the category of heterocyclic compounds that constitute the majority of pharmaceutically important drugs and drug candidates, and they are adaptable to highly stereoselective syntheses and further elaboration. New chemical space is accessed catalytically from stable diazo compounds and affords structurally complex products with high efficiencies and selectivities. Our research is being developed in stages in order to assess biological effectiveness: (1) development of unique libraries that explore new chemical space, (2) biological assays of representative compounds, and (3) structural modifications that enhance biological activities.
Enoldiazoacetates are a subclass of vinyldiazoacetates that are directly accessed from diazoacetoacetates in high yield. These compounds are exceptionally stable, having no tendency to undergo intramolecular dipolar cycloaddition to form 3H-pyrazoles, and they have shown remarkable versatility in catalytic cycloaddition reactions. Their intermolecular cycloaddition reactions have made possible facile synthesis of heterocyclic compounds containing one or more nitrogen and/or oxygen atoms (Scheme 1). With enoldiazoacetates we have shown that stepwise highly enantioselective [3+3]-cycloaddition is a viable synthetic transformation for the construction of six-membered ring heterocyclic compounds, and a variety of [3+2]-cycloaddition processes give five-membered ring heterocycles. The key to the rapid development of this methodology has been recognition that catalytically generated metallo-enolcarbenes are effective dipolar adducts for [3+3]-cycloaddition. One or more heteroatoms is introduced and, with high levels of enantioselectivity obtained via asymmetric catalysis, this methodology has the potential to be the catalytic method of choice for the synthesis of many classes of heterocyclic compounds.
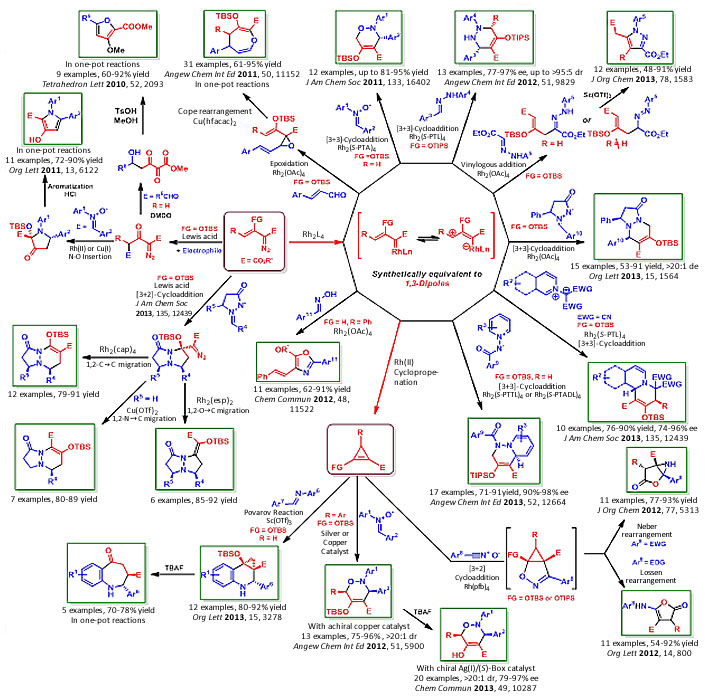
Scheme 1
Expanding Chemical Space for Heterocyclic Compounds through Applications of Enol-diazoacetates. Asymmetric catalytic syntheses of 1,2-Oxazines, 1,2,3,6-Tetrahydropyridazines, Dihydro-quinolines, and Quinolizidines. Catalytic Selective Syntheses of Pyrazolidinones, Pyrazoles, Pyrazolidin-ones, Tetrahydroquinolines, Benzazepines, Aminofuranones, Aziridines, and Oxazoles, among others.

