In the long history of Diels-Alder reactions there has not been a reported example of [4+2]-cycloaddition between a diene and a diazo compound. Their isoelectronic allene and ketene counterparts, however, do undergo these reactions and have received considerable attention (Scheme 3). Concerted thermal [4+2]-cycloaddition reactions of dienes with highly polarized allenes have been extensively investigated, but favorable competing stepwise [2+2]-cycloaddition reactions diminish their synthetic importance. Transition-metal-catalyzed allene-diene cycloaddition reactions, although recent in their discovery, have circumvented this competition and become a mainstay of new process developments. Ketenes readily undergo [2+2]-cycloaddition5 and, recently, [4+2]-cycloaddition reactions, but for the latter only with highly activated diene equivalents6 or with the aid of suitable catalysts. In contrast, diazo compounds commonly react with one double bond of dienes by dipolar cycloaddition or, following dinitrogen extrusion, by cyclopropanation (Scheme 1), but not by [4+2]-cycloaddition.
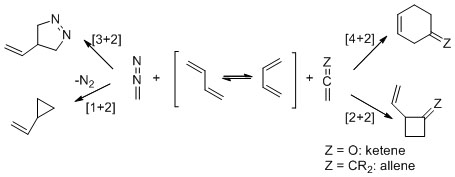
Scheme 1
Reactions of dienes with diazo compounds, allenes and ketenes.
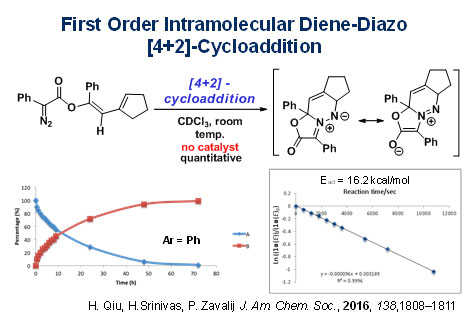
The diene is formed by gold(I) catalysed rearrangement of propargyl phenyldiazoacetates in the presence of a pyridine-N-oxide. Isolation of the diene product allows examination of the cycloaddition reaction that occurs at room temperature. This process is favored over [3+2]-cycloaddition only for this system, and this has been confirmed by computational methods. The heterocyclic product is a stable dipole that itself undergoes cycloaddition with activated alkenes.