Oxidation reactions of organic compounds are critically important throughout the chemical sciences, and they are particularly relevant in biological systems and for industrial processes. The search for precise control over the oxidation state of functional groups in target molecules and for chemo- and regioselectivity of oxidation has led to the development of a large variety of oxidative protocols. Variation of selectivity and improvement of efficiency has inspired progress in catalytic approaches, and efforts to decrease cost have drawn investigators to chemical catalysis and towards dioxygen as the optimal oxidant. Peroxides, especially hydroperoxides, have been useful for the development of selective oxidations and for understanding how to eventually engage dioxygen. We have made substantial progress in developing and understanding radical oxidations in their investigations using dirhodium caprolactamate [Rh2(cap)4] as a catalyst and tert-butyl hydroperoxide (TBHP) as a terminal oxidant (Scheme 1). Recent research on dirhodium caprolactamate catalyzed chemical oxidations with hydroperoxides has not only produced highly selective processes (for example, allylic oxidation – the Umera-Doyle reaction, oxidative Mannich reactions, and phenolic peroxidation), but recently we have also uncovered a synthetically applicable iron(III) chloride catalyzed oxidative Mannich reaction in which molecular oxygen is the oxidant.
Scheme 1
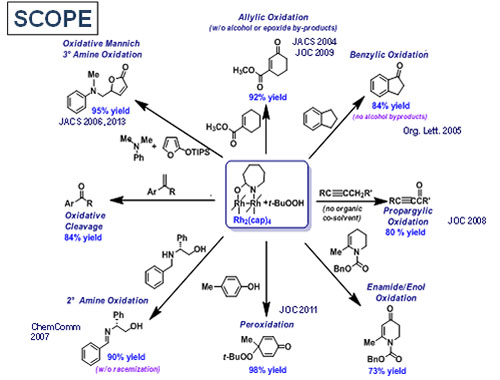
The Rh2(cap)4–TBHP system has provided multiple synthetically-valuable oxidative transformations and continues to be a source for fruitful discoveries.
A wide range of oxidative processes employs tert-butyl hydroperoxide (TBHP) as a terminal oxidant. With a half-life of over 520 hours in refluxing benzene, TBHP has fewer handling risks than does H2O2 in water or peracetic acid. The low acidity of TBHP (pKa = 12.8) contributes to the versatility of its uses, and volatile reaction products derived from TBHP (tert-butyl alcohol, water, molecular oxygen, and di-tert-butyl peroxide) simplify purification. Anhydrous solutions of TBHP are commercially available or can be easily prepared due to the high solubility of TBHP in organic solvents. Furthermore, TBHP is available as a 70% aqueous solution (T-HYDRO) that offers fewer handling risks and is less expensive than solutions of TBHP decane or benzene.
Sulfoxides with catalytic dirhodium(II) is a highly chemoselective reagent for the quantitative conversion of diazocarbonyl compounds to dicarbonyl compounds without also oxidizing other unsaturated centers. This oxidative procedure will be elaborated for the conversion of diazoacetoacetates to 2,3-diketoesters that have shown remarkable reactivity and selectivity in asymmetric ene reactions41 and show promise for other condensation reactions.

