Our current research interest focuses mainly in the following areas
Catalytic Asymmetric Organocatalysis
We are interested in developing novel organocatalytic catalytic asymmetric synthesis of chiral molecules, especially those with potential biological activities. Using proline- and cinchona-based organocatalysts, our group have developed many highly stereoselective methods for the synthesis of chiral molecules and scaffolds that are of potential biological activities and/or highly useful in organic synthesis. Some of these molecules are collected below in Scheme 1.
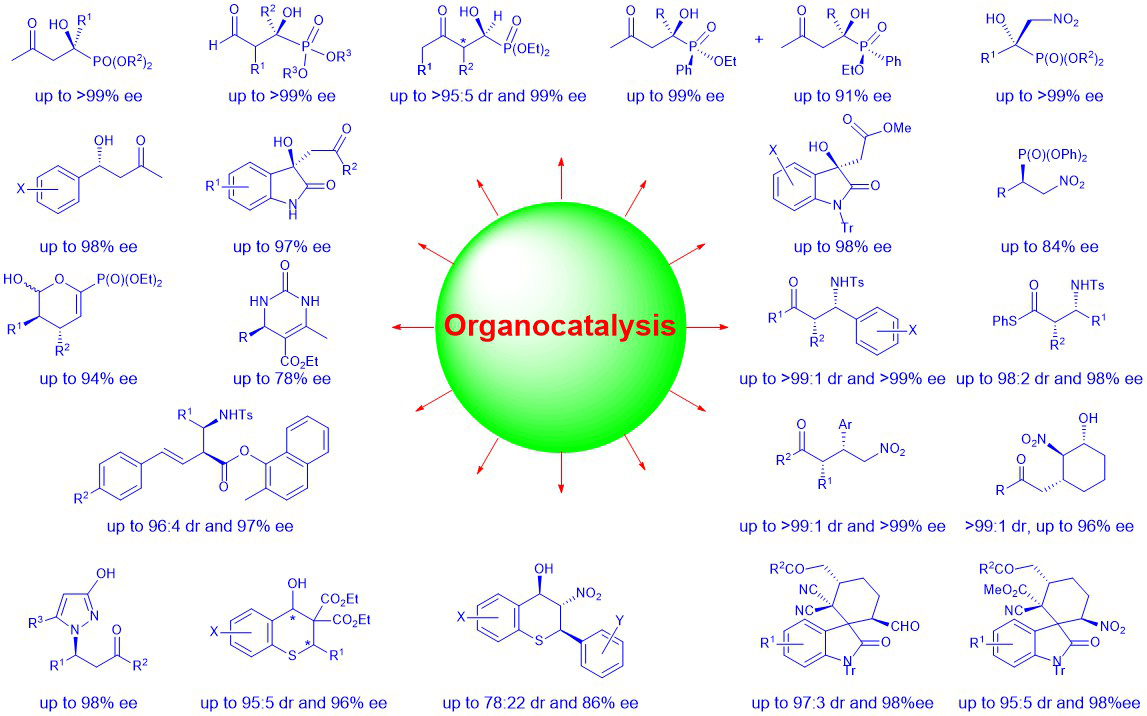
Scheme 1: Selected chiral molecules synthesized in our lab using organocatalytic methods
Since catalyst plays a crucial role in a catalytic asymmetric reaction, we are also interested in developing better methods for the easy synthesis and modification of organocatalysts. In this aspect, we have developed the modularly designed organocatalysts (MDOs), which can self-assemble from the precatalyst modules (amino acids and cinchona alkaloid-derived (thio)ureas or squaramides (Scheme 2). The formation and modification of the organocatalyst can be simply achieved by mixing the desired precatalyst modules in the reaction media. These MDOs have been shown to be more effective catalysts (higher reactivity and stereoselectivity) than the individual precatalysts in asymmetric organocatalytic reactions.
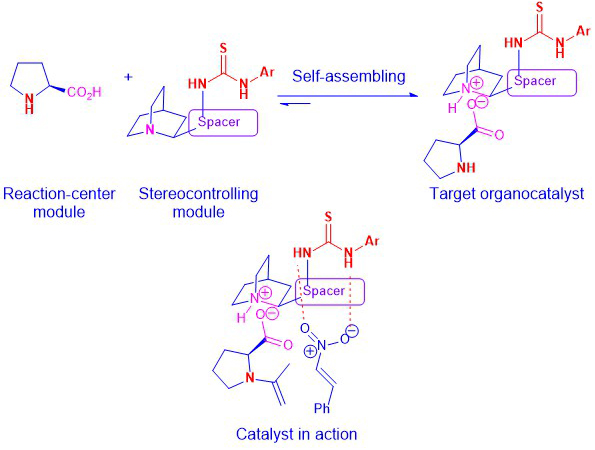
Scheme 2: Formation of MDOs via the self-assembly of precatalyst modules
Obtaining different diastereomers from the same substrates in high stereoselectivities is much more challenging than obtaining different enantiomers from the same substrates. While there are several methods allow the synthesis different diastereomers from the same substrates, diastereodivergent catalysis is the most efficient way for obtaining multiple diastereomers from the same substrates. Taking the advantage of the properties of the MDOs, which allow us to obtain pseudo-diastereomeric catalysts or even completely different catalysts easily through the self-assembly of the desired precatalyst modules, we are keen to developing novel diastereodivergent organocatalytic methods for organic synthesis (Scheme 3).
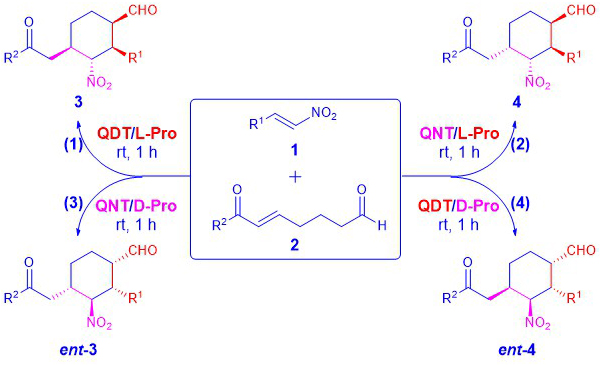
Scheme 3: Diastereodivergent catalysis with MDOs
Novel Photochemical Reactions Based on the EDA Complexes
Even if the two components forming the electron-donor-acceptor (EDA) complex do not absorb visible light, the resulting EDA complex often does, and this property can be leveraged for visible-light-driven radical synthetic chemistry, which offers an opportunity to conduct photochemical reactions in a convenient and sustainable manner (Scheme 4). We are interested in developing novel synthetic methods based on the halogen bond complexes and EDA complexes, especially those of N-haloimides (Scheme 4).
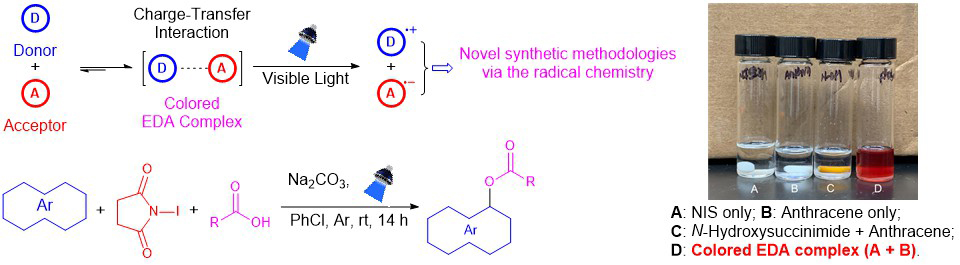
Scheme 4: Sustainable visible-light-driven reactions of the EDA complexes
Medicinal Chemistry
Through collaborations, we are interested in testing and improving the potential biological activities of the new compounds synthesized by the methodologies developed in our group. We are also interested in synthesizing drug conjugates for tumor-selective targeting. For example, we have developed a drug conjugate based on the click chemistry of azide and acrolein for cancer suppression (Scheme 5). Acrolein exists in high concentration in cancer cells due to abnormal cancer cell metabolism and oxidative stress, but not in normal cells and, therefore, our synthesized conjugate will selectively release the drug molecule in cancer cells to achieve a tumor-selective targeting (Scheme 5).
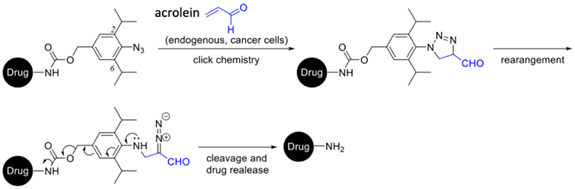
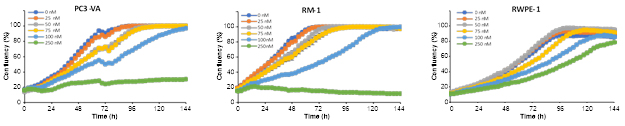
Scheme 5: Synthesis of drug conjugates for tumor-selective anti-cancer study [The synthesized conjugate inhibits the growth of the prostate cancer cell lines (PC3-VA and RM-1) but not that of normal cells (RWPE-1)]

