Research at the Interface of Chemistry and Biology
NEW SYNTHETIC METHODS AND CATALYSIS
Pd-Catalyzed Synthesis of Substituted Allenes
The discovery and development of new catalytic asymmetric methodologies remains a fundamental challenge and priority in organic synthesis. Despite the incredible repertoire of chiral catalysts available (either metal-based or carbon-based) and the increasing ability to rationally design new ones, critical gaps still remain in several areas of asymmetric synthesis including the catalytic construction of axial chirality. Nowhere is this gap more obvious than in the synthesis of chiral allenes. My group has a long-term goal to exploit metal-mediated beta-hydride eliminations as a powerful new approach in synthetic methodology and asymmetric catalysis. Towards this goal, we have developed several Pd-catalyzed syntheses of substituted allenes that are highlighted above in Figure 1. Early work involved the use of stereodefined enol triflates to access substituted allenoates in both racemic and enantioenriched forms. Our current work involves the discovery and development of the alkynyl Heck reaction as one of the most direct syntheses of allenes known. Key to the success of this approach was the design of a new hybrid Pd(0)-catalyst, BobCat, that was invented in our labs here at UTSA.
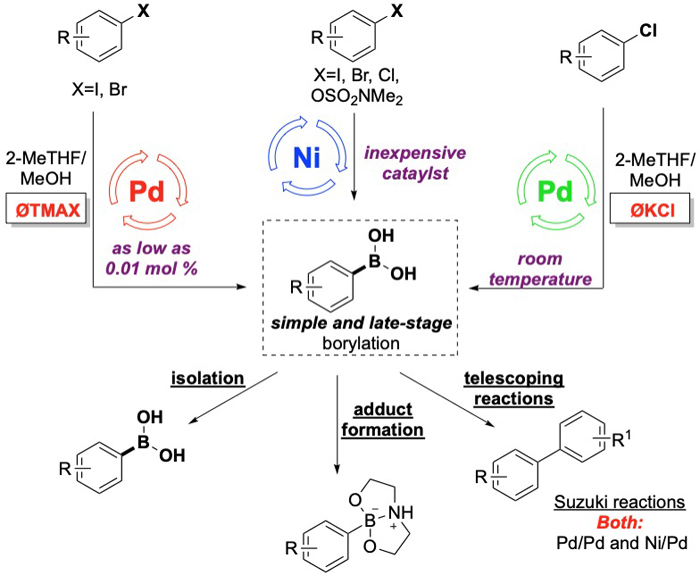
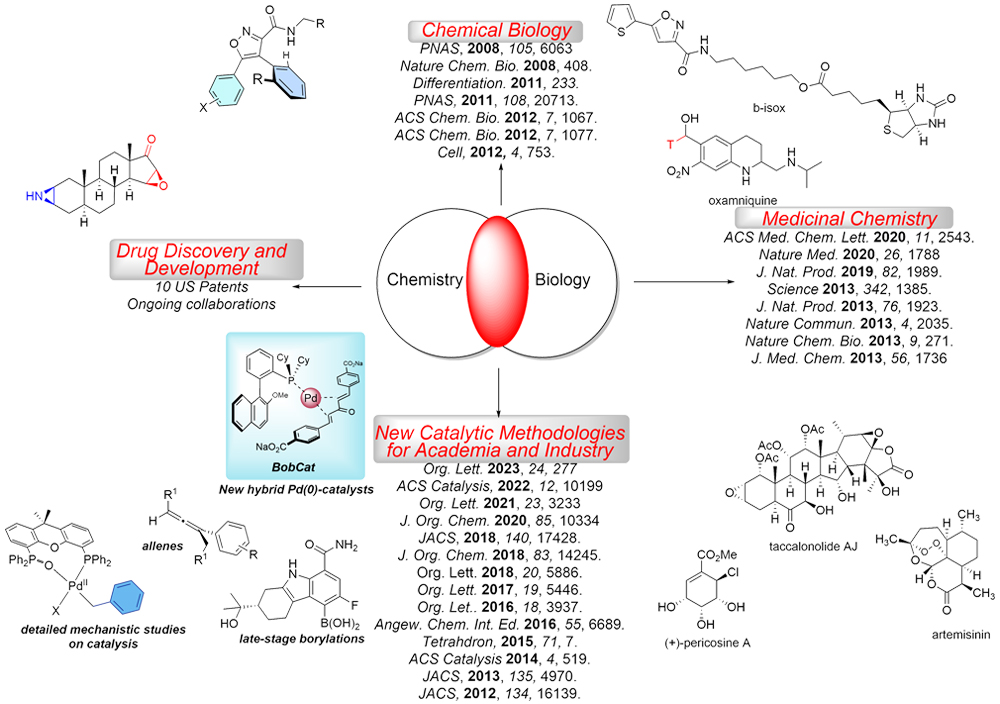
Developing Highly Efficient Metal-Catalyzed Direct Borylation and Cross-Coupling Reactions
For several years now, we have developed a fruitful collaboration with the Chemical Synthesis & Development (CSD) group at BMS. Our latest efforts are focusing on developing a "users-guide" to Pd and Ni-catalyzed borylations and subsequent Suzuki cross-coupling reactions. Key to this effort is the identification of optimized reaction parameters that provide high conversion at very low catalyst loadings (i.e., ‹ 1 mol %) for the borylation step, the Suzuki reaction, or both. At these low catalyst loadings, we are also pursuing "late-stage" borylations and Suzuki reaction to functionalize complex molecules and APIs.
DRUG DISCOVERY AND MEDICINAL CHEMISTRY
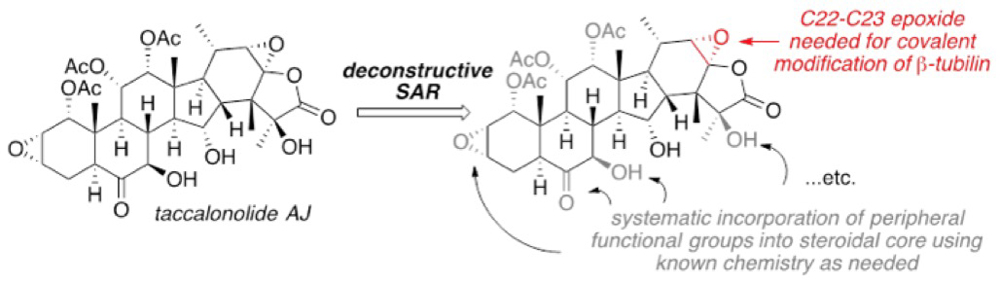
Deconstructive Structure-Activity Relationship (SAR) Studies of the Taccalonolides
In collaboration with Dr. April Risinger at UT Health San Antonio, we are developing new synthetic routes to simplified analogs of the taccalonolides as potential new treatments for triple negative breast cancer. Naturally occurring taccalonolides (and semi-synthetic derivatives) are highly complex, steroid-based natural products that have proven microtubule-stabilizing activity that is complementary to the taxanes yet overcome various mechanisms of resistance. As such, they are attractive targets for cancer drug discovery yet their complexity precludes their pre-clinical development beyond academic endeavors. Thanks to funding from the Cancer Prevention and Research Institute of Texas (CPRIT), we have synthesized simplified analogs of the taccas that demonstrate promising cytotoxic profiles against various cancer cells lines. Our continuing efforts are focused on developing new analogs that contain the entire carbon framework of the taccas that are synthetically tractable and exhibit microtubule stabilizing activity relevant to pursue their pre-clinical development.

