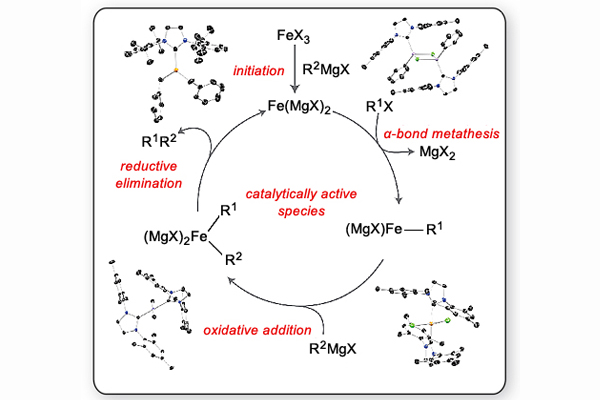
Earth-Abundant Metal Catalysis
Unlike the better-understood features of oxidative addition and reductive elimination with Group 10 metals such as palladium, the details of M-C and C-C bond formation are not well understood with Earth-abundant metals such as iron. We seek to probe the mechanisms of non-precious metal catalysts through the synthesis of new organometallic complexes and an examination of their reactivity. We are particularly interested in the roles that redox chemistry, β-hydride elimination, and intermetallic complexes play in the catalytic mechanism. Iron catalysts are of great potential value due to their promise as more sustainable and environmentally benign alternatives to their heavy metal counterparts.
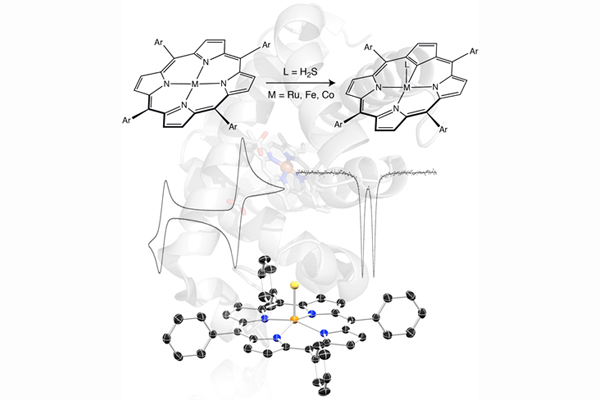
Bioinorganic Chemistry
Nature is remarkable in its ability to efficiently use and regulate small molecules that would otherwise be toxic in large concentrations. We are interested in exploring the chemistry of transition-metal compounds containing these biologically relevant small molecules, namely NO and H2S. By modeling the active sites of various metalloenzymes for which these molecules may serve as metabolites, signaling agents, or pathogens, we hope to gain insights into their fundamental mechanisms of action in biology. At the heart of this investigation is an understanding of the basic coordination chemistry of these molecules and how these ligands are perturbed upon modifications to the metal center (redox events, ligand substitution, etc.).
Coordination Chemistry
The design of new supporting ligands is a continuing area of interest in inorganic chemistry. Such ligands can modulate metal centered reactivity, redox potential, and coordination number. We are interested in exploring new pincer ligands based on a central pyrrole moiety. Such compounds represent an interesting counterpoint to the well-studied class of pincer ligands based on pyridines. Furthermore, the uni-negative pyrrolide group is a poor π-donor, allowing the ligand to bind to a variety of early and late metals. We are interested in exploiting these new pincer-based compounds for catalysis and small molecule activation.

